Detail
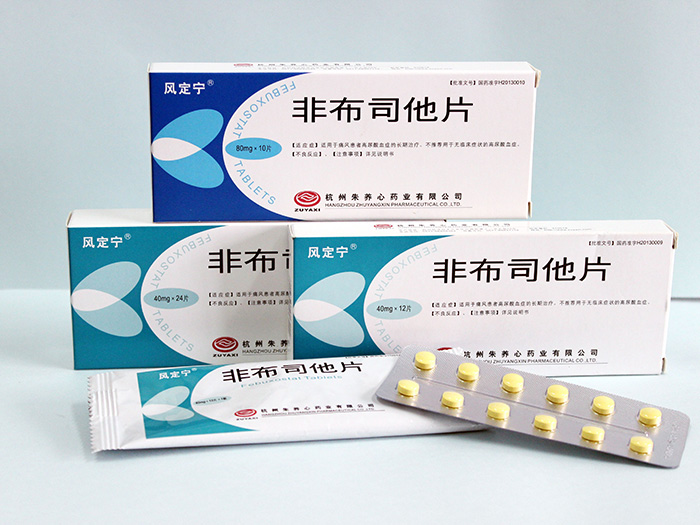
Feng Ding Ning (Febuxostat tablets)——The world's expected new drug in 40 years
● First listed ● Patent protection ● Excellent quality
Concise prescription data
|
Generic name |
Febuxostat tablets |
|
Specifications |
(1)40mg (2)80mg |
|
Ingredients |
The active ingredient of this product is febuxostat. |
|
Indications |
It is suitable for long-term treatment of hyperuricemia in gout patients. |
|
Usage and dosage |
The recommended oral dose is 40mg or 80mg, once a day. The starting dose is 40mg, once a day. If the serum uric acid level does not fall below 360 μmol/L after 2 weeks, the recommended dose is increased to 80mg, once a day. When administered, there is no need to consider the effects of food and antacids. |
|
Adverse reactions |
See the instructions for details. |
|
Taboo |
This product is contraindicated in patients undergoing azathioprine and purine purine therapy. |
|
Use in children |
The safety and efficacy of this product for patients under the age of 18 have not been determined. |
|
Use in the elderly |
Elderly patients do not need to adjust the dose. |
|
Storage |
Tightly sealed and kept in shading place below 25ºC. |
|
Packing |
Aluminum blister packaging, with composite bag outside. 80mg: |
|
Shelf life |
48 months. |
| Standard | YBH00432013 |
| Approval No. |
40mg: SFDA approval number H20130009 |